|

28
July 2023
By Bob Yirks
A life-cycle assessment of the
production of hydrogen from natural subsurface accumulations
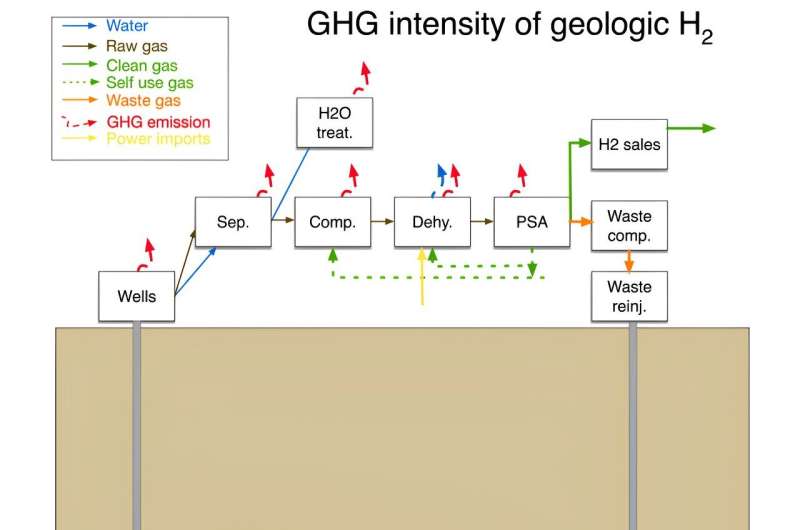
Credit: Joule (2023). DOI:
10.1016/j.joule.2023.07.001
Adam Brandt, an energy science and environmental
engineer at Stanford University, has conducted a life-cycle assessment
of hydrogen production from natural subsurface accumulations, also
known as geological hydrogen extraction, to determine whether the idea
is practical. In his paper, published in the journal Joule, he looks
at factors involved in extracting geological hydrogen, such as the
impact on climate change and how much of it is available for
extraction.
As the world continues to warm (July 2023 is on course to be
the hottest month ever recorded) scientists are looking for ways to
replace greenhouse-gas-producing energy sources with those that are
more friendly to the environment. One such approach involves the use
of hydrogen gas as a fuel source.
Hydrogen is considered to be a "green" type of gas because it
produces only water when burned. Unfortunately, the most popular means
for producing hydrogen involves burning natural gas, which does
produce greenhouse gas emissions. For that reason, scientists have
been looking for other ways to obtain hydrogen. One such process
involves extracting geological hydrogen from the ground, an approach
that has not received much attention until recently. In this new
effort, Brandt looks at the factors involved in extracting hydrogen
from underground sources.
In his analysis, Brandt focuses most heavily on whether
extracting hydrogen is more or less harmful to the environment. He
notes that because the idea of extracting hydrogen for commercial use
is still so new, it is still not clear if it is a feasible
proposition. As an example, he notes that excavation operations would
lead to some leakage of the gas into the atmosphere. And while
hydrogen is not a greenhouse gas, it does react with other greenhouse
gases, resulting in an increase in their lifespan.
Another problem is that geological hydrogen is rarely pure—most
of the time, it is mixed with methane, nitrogen or other gases, all of
which would have to be removed before use. Such processing would
require energy, which might lead to greenhouse gas emissions. He also
notes that, to date, researchers don't know whether there is enough
geological hydrogen available for harvesting.
Brandt concludes that, despite the hurdles involved, it appears
that more research into the idea of geological hydrogen extraction is
warranted. At its worst, his analysis showed, it would likely be no
worse than "green hydrogen," which is made by splitting water
molecules.
Green Play Ammonia™, Yielder® NFuel Energy.
Spokane, Washington. 99212
509 995 1879
Cell, Pacific Time Zone.
General office:
509-254
6854
4501 East Trent
Ave.
Spokane, WA 99212
|