|

18
August 2023
By
Nathi Magubane, University
of Pennsylvania
Researchers develop new
carbon-capture solution for a cleaner, more energy-dense fuel source
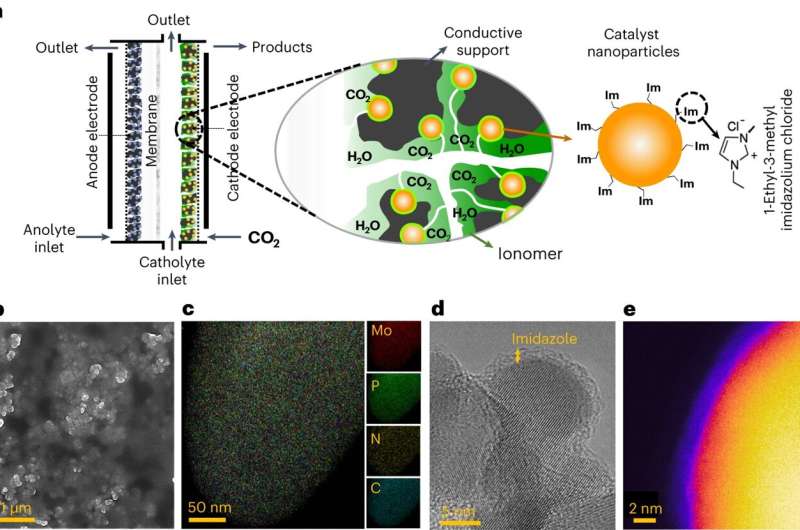
Characterizations of the catalyst
microenvironment of the developed ImF-Mo3P electrocatalytic system
studied in a flow electrolyser. a, Schematic of the catalyst
microenvironment composed of Mo3P nanoparticles covered by an Im layer
coated with an anion-exchange ionomer and deposited on a conductive
carbon support. b, SEM image of the cathode electrode coated on the
gas diffusion layer. c, TEM-EDS mapping image of the catalyst
microenvironment. EDS mapping of Mo, P, N and C elements are shown
with red, green, yellow and blue colors, respectively. d, TEM image of
the dispersed nanoparticles in the catalyst microenvironment. e, False
color EELS image of a single ImF-Mo3P nanoparticle. This image
suggests an Im layer of about 1 nm. Credit: Nature Energy (2023). DOI:
10.1038/s41560-023-01314-8
Over the past three centuries, especially since
the Industrial Revolution in the late 18th and 19th centuries, human
activities have significantly increased greenhouse gas levels in the
Earth's atmosphere. The main culprits are fossil fuel consumption,
industrial processes, deforestation, and waste management.
In response, the United States aims to cut greenhouse gas
emissions by 50 to 52% from 2005 levels by 2030. This initiative
aligns with a global effort to achieve net-zero greenhouse gas
emissions by 2050. With electric power and industry sectors
contributing to about half of U.S. carbon dioxide (CO2) emissions,
finding solutions in these areas is imperative.
Now, in a paper published in Nature Energy, researchers from
the University of Pennsylvania, Illinois Institute of Technology, and
the University of Illinois at Chicago have developed a system that can
convert CO2 emissions into propane (C3H8), a cleaner, more
energy-dense fuel source.
"Electrochemical conversion of CO2 can serve future energy
needs by storing renewable energy and closing the anthropogenic carbon
cycle," says co-author Andrew Rappe of the School of Arts & Sciences
at Penn. "This research paves the way to new solutions that will
tackle energy storage challenges and meaningfully reduce CO2 levels."
"Making renewable chemical manufacturing is really important,"
says co-author Mohammad Asadi of Illinois Institute of Technology.
"It's the best way to close the carbon cycle without losing the
chemicals we currently use daily."
Copper has traditionally been the go-to element for researchers
investigating efficient ways to convert CO2 into valuable chemicals
and fuels, both to curb its environmental impact and provide new
energy storage solutions. However, the fuels produced have been
low-energy density single-carbon compounds like methane.
"Getting energy-dense multi-carbon products like C3H8 has
remained a challenge due to the many intermediates that form
throughout the chemical conversion process," explains Zhen Jiang,
co-first author of the paper and a former postdoctoral researcher in
The Rappe Group. "Additionally, most strategies to increase a
material's selectivity for multi-carbon molecules tend to be
energetically costly."
Jiang says that the team sought ways to move beyond existing
catalysts like copper—and their modest selectivity for multi-carbon
products or their sluggish kinetics—and investigated ways to add ionic
liquid (IL) into the catalytic system. This prompted the team to look
at tri-molybdenum phosphide (Mo3P) as the catalytic material.
"Based on our theoretical simulations, we found that the IL
layer can enhance the adherence of CO2 and subsequent groups during
reaction on the Mo3P catalyst surface, thus stabilizing the
intermediates at different sites along the surface to produce C3H8
with an unparalleled efficiency of 91%," says Jiang.
The team also notes that this key finding led to a new paradigm
for exploring the relationship between materials in electrocatalytic
systems.
"Conventionally, the solid-state catalyst, and the aqueous
solution that bridges ion transfer throughout the reaction acted with
less mutual promotion at the interface," says Jiang. "But now, we can
apply a hybrid approach via techniques like IL coating on solid-state
catalysts and re-examine previously tried systems with our novel
understanding of the catalyst's microenvironment."
Looking ahead, the researchers plan to build on this research
in two ways: one, to develop a catalog of ionic liquids and their
effectiveness in fuel-generating catalysts and other electrochemical
systems; and two, investigate new catalysts for the conversion of CO2
into more energy-dense fuel sources from fuel gas to light oil with
more carbon atoms.
Rappe says, "Extending this research to higher-weight
hydrocarbons could close the carbon cycle by creating natural gas,
propane, gasoline, and even jet fuel directly from the CO2 made by
previous fuel combustion. In this way, the same carbon atoms store
energy over and over, and we don't release them into the atmosphere."
Green Play Ammonia™, Yielder® NFuel Energy.
Spokane, Washington. 99212
509 995 1879
Cell, Pacific Time Zone.
General office:
509-254
6854
4501 East Trent
Ave.
Spokane, WA 99212
|